| Title | Targeting the polyamine pathway with transition-state analogue inhibitors of 5'-methylthioadenosine phosphorylase. |
| Publication Type | Journal Article |
| Year of Publication | 2004 |
| Authors | Evans GB, Furneaux RH, Schramm VL, Singh V, Tyler PC |
| Journal | J Med Chem |
| Volume | 47 |
| Issue | 12 |
| Pagination | 3275-81 |
| Date Published | 2004 Jun 03 |
| ISSN | 0022-2623 |
| Keywords | Adenine, Humans, Polyamines, Purine Nucleosides, Purine-Nucleoside Phosphorylase, Pyrimidinones, Pyrroles, Pyrrolidines, Stereoisomerism, Structure-Activity Relationship |
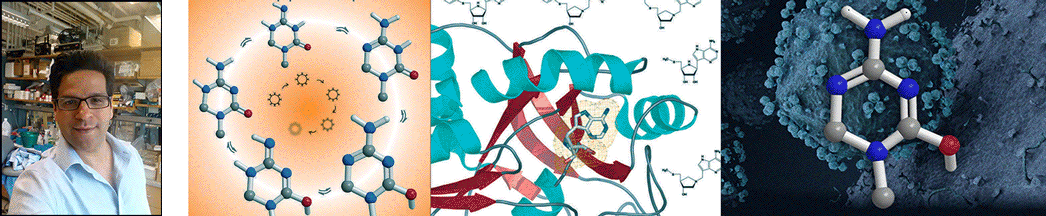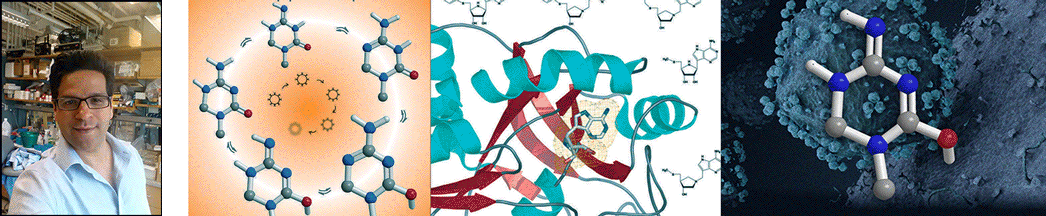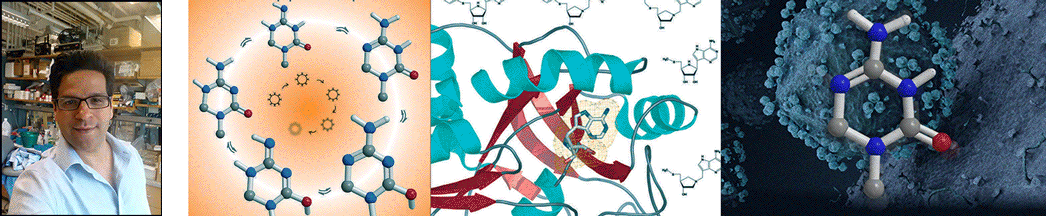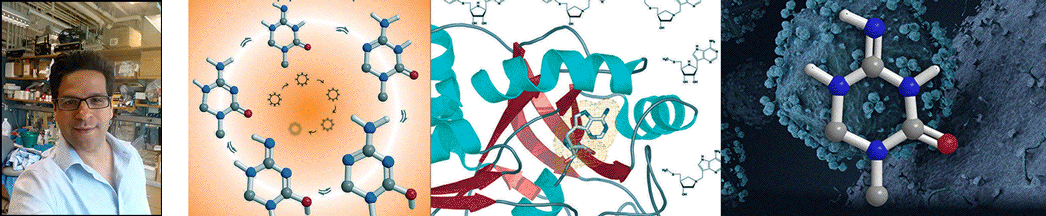
| Abstract | The polyamine biosynthetic pathway is a therapeutic target for proliferative diseases because cellular proliferation requires elevated levels of polyamines. A byproduct of the latter stages of polyamine biosynthesis (the synthesis of spermidine and spermine) is 5'-methylthioadenosine (MTA). In humans, MTA is processed by 5'-methylthioadenosine phosphorylase (MTAP) so that significant amounts of MTA do not accumulate. Potent inhibitors of MTAP might allow the buildup of sufficient levels of MTA to generate feedback inhibition of polyamine biosynthesis. We have designed and synthesized a family of potential transition-state analogue inhibitors of MTAP on the basis of our knowledge of the transition-state structure of purine nucleoside phosphorylase and the assumption that it is likely the two enzymes share a common catalytic mechanism. Several of the inhibitors display slow-onset tight-binding properties, consistent with them being transition-state analogues, with the most potent having a dissociation constant of 166 pM. |
| DOI | 10.1021/jm0306475 |
| Alternate Journal | J Med Chem |
| PubMed ID | 15163207 |
Targeting the polyamine pathway with transition-state analogue inhibitors of 5'-methylthioadenosine phosphorylase.
Submitted by Vipender Singh on