| Title | Targeting a novel Plasmodium falciparum purine recycling pathway with specific immucillins. |
| Publication Type | Journal Article |
| Year of Publication | 2005 |
| Authors | Ting L-M, Shi W, Lewandowicz A, Singh V, Mwakingwe A, Birck MR, Ringia EATaylor, Bench G, Madrid DC, Tyler PC, Evans GB, Furneaux RH, Schramm VL, Kim K |
| Journal | J Biol Chem |
| Volume | 280 |
| Issue | 10 |
| Pagination | 9547-54 |
| Date Published | 2005 Mar 11 |
| ISSN | 0021-9258 |
| Keywords | Adenine, Adenosine Deaminase, Amino Acid Sequence, Animals, Conserved Sequence, Escherichia coli, Humans, Hypoxanthine, Inosine, Methylthioinosine, Molecular Sequence Data, Plasmodium falciparum, Purine-Nucleoside Phosphorylase, Purines, Sequence Alignment, Sequence Homology, Amino Acid |
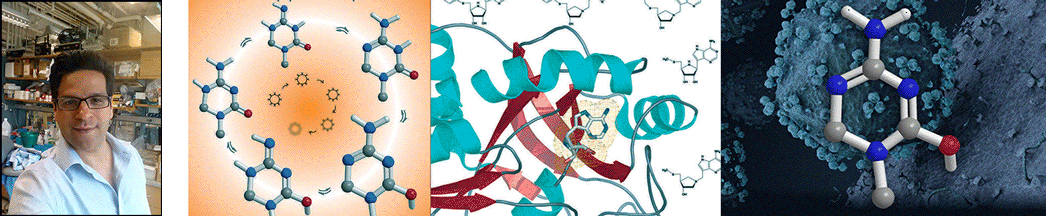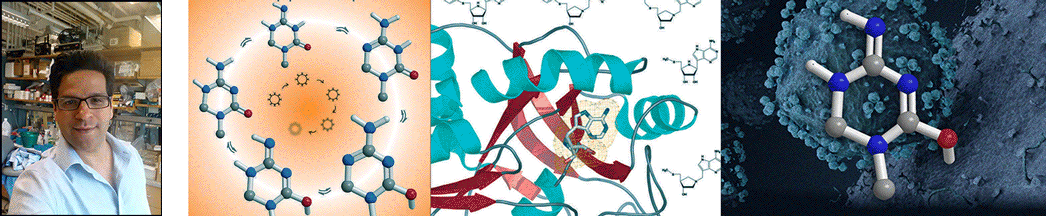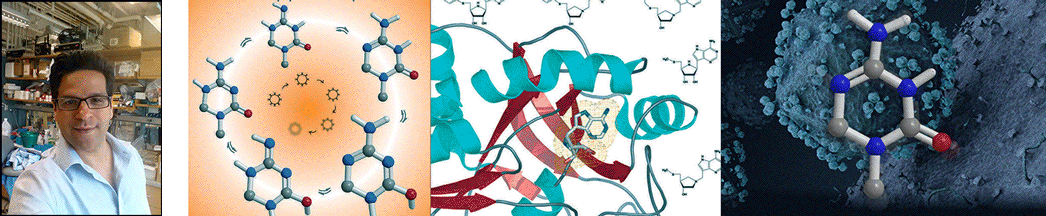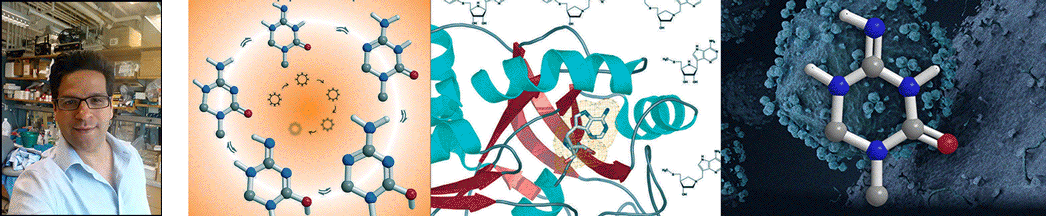
| Abstract | Plasmodium falciparum is unable to synthesize purine bases and relies upon purine salvage and purine recycling to meet its purine needs. We report that purines formed as products of polyamine synthesis are recycled in a novel pathway in which 5'-methylthioinosine is generated by adenosine deaminase. The action of P. falciparum purine nucleoside phosphorylase is a convergent step of purine salvage, converting both 5'-methylthioinosine and inosine to hypoxanthine. We used accelerator mass spectrometry to verify that 5'-methylthioinosine is an active nucleic acid precursor in P. falciparum. Prior studies have shown that inhibitors of purine salvage enzymes kill malaria, but potent malaria-specific inhibitors of these enzymes have not been described previously. 5'-Methylthio-immucillin-H, a transition state analogue inhibitor that is selective for malarial relative to human purine nucleoside phosphorylase, kills P. falciparum in culture. Immucillins are currently in clinical trials for other indications and may also have application as anti-malarials. |
| DOI | 10.1074/jbc.M412693200 |
| Alternate Journal | J Biol Chem |
| PubMed ID | 15576366 |
Targeting a novel Plasmodium falciparum purine recycling pathway with specific immucillins.
Submitted by Vipender Singh on