| Title | Structure and inhibition of a quorum sensing target from Streptococcus pneumoniae. |
| Publication Type | Journal Article |
| Year of Publication | 2006 |
| Authors | Singh V, Shi W, Almo SC, Evans GB, Furneaux RH, Tyler PC, Painter GF, Lenz DH, Mee S, Zheng R, Schramm VL |
| Journal | Biochemistry |
| Volume | 45 |
| Issue | 43 |
| Pagination | 12929-41 |
| Date Published | 2006 Oct 31 |
| ISSN | 0006-2960 |
| Keywords | Amino Acid Sequence, Binding Sites, Catalysis, Crystallography, X-Ray, Enzyme Activation, Escherichia coli, Kinetics, Models, Molecular, Molecular Sequence Data, N-Glycosyl Hydrolases, Protein Binding, Protein Structure, Secondary, Protein Structure, Tertiary, Ribitol, Sequence Analysis, Protein, Signal Transduction, Streptococcus pneumoniae, Structural Homology, Protein, Substrate Specificity |
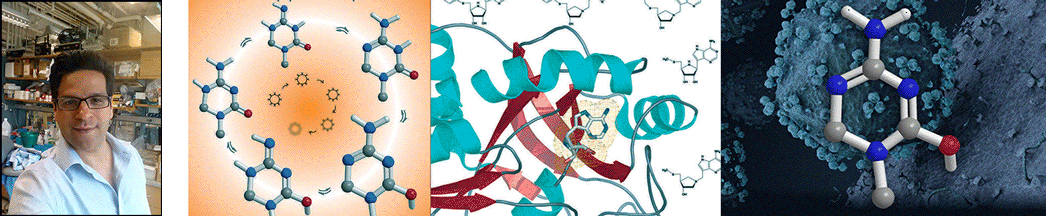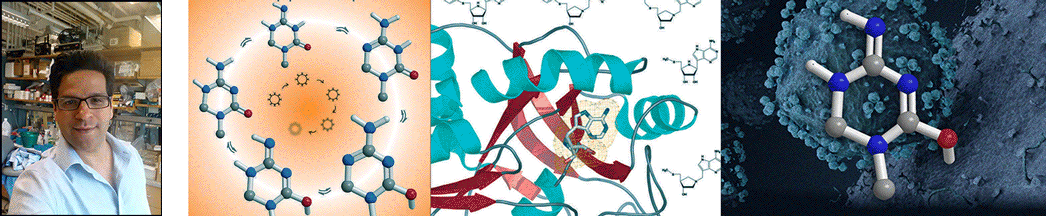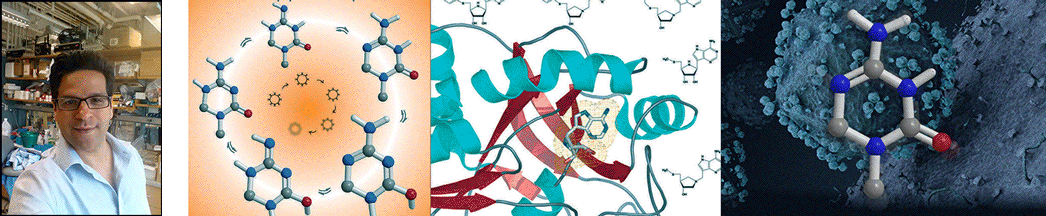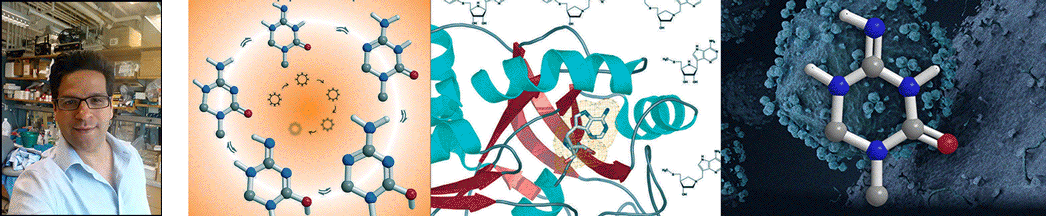
| Abstract | Streptococcus pneumoniae 5'-methylthioadenosine/S-adenosylhomocysteine hydrolase (MTAN) catalyzes the hydrolytic deadenylation of its substrates to form adenine and 5-methylthioribose or S-ribosylhomocysteine (SRH). MTAN is not found in mammals but is involved in bacterial quorum sensing. MTAN gene disruption affects the growth and pathogenicity of bacteria, making it a target for antibiotic design. Kinetic isotope effects and computational studies have established a dissociative S(N)1 transition state for Escherichia coli MTAN, and transition state analogues resembling the transition state are powerful inhibitors of the enzyme [Singh, V., Lee, J. L., Núñez, S., Howell, P. L., and Schramm, V. L. (2005) Biochemistry 44, 11647-11659]. The sequence of MTAN from S. pneumoniae is 40% identical to that of E. coli MTAN, but S. pneumoniae MTAN exhibits remarkably distinct kinetic and inhibitory properties. 5'-Methylthio-Immucillin-A (MT-ImmA) is a transition state analogue resembling an early S(N)1 transition state. It is a weak inhibitor of S. pneumoniae MTAN with a K(i) of 1.0 microM. The X-ray structure of S. pneumoniae MTAN with MT-ImmA indicates a dimer with the methylthio group in a flexible hydrophobic pocket. Replacing the methyl group with phenyl (PhT-ImmA), tolyl (p-TolT-ImmA), or ethyl (EtT-ImmA) groups increases the affinity to give K(i) values of 335, 60, and 40 nM, respectively. DADMe-Immucillins are geometric and electrostatic mimics of a fully dissociated transition state and bind more tightly than Immucillins. MT-DADMe-Immucillin-A inhibits with a K(i) value of 24 nM, and replacing the 5'-methyl group with p-Cl-phenyl (p-Cl-PhT-DADMe-ImmA) gave a K(i) value of 0.36 nM. The inhibitory potential of DADMe-Immucillins relative to the Immucillins supports a fully dissociated transition state structure for S. pneumoniae MTAN. Comparison of active site contacts in the X-ray crystal structures of E. coli and S. pneumoniae MTAN with MT-ImmA would predict equal binding, yet most analogues bind 10(3)-10(4)-fold more tightly to the E. coli enzyme. Catalytic site efficiency is primarily responsible for this difference since k(cat)/K(m) for S. pneumoniae MTAN is decreased 845-fold relative to that of E. coli MTAN. |
| DOI | 10.1021/bi061184i |
| Alternate Journal | Biochemistry |
| PubMed ID | 17059210 |
| PubMed Central ID | PMC2517848 |
| Grant List | R01 GM041916-20 / GM / NIGMS NIH HHS / United States P41-EB-01979 / EB / NIBIB NIH HHS / United States R37 GM041916 / GM / NIGMS NIH HHS / United States GM41916 / GM / NIGMS NIH HHS / United States R01 GM041916 / GM / NIGMS NIH HHS / United States |
Structure and inhibition of a quorum sensing target from Streptococcus pneumoniae.
Submitted by Vipender Singh on