| Title | Femtomolar transition state analogue inhibitors of 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase from Escherichia coli. |
| Publication Type | Journal Article |
| Year of Publication | 2005 |
| Authors | Singh V, Evans GB, Lenz DH, Mason JM, Clinch K, Mee S, Painter GF, Tyler PC, Furneaux RH, Lee JE, P Howell L, Schramm VL |
| Journal | J Biol Chem |
| Volume | 280 |
| Issue | 18 |
| Pagination | 18265-73 |
| Date Published | 2005 May 06 |
| ISSN | 0021-9258 |
| Keywords | Deoxyadenosines, Enzyme Inhibitors, Escherichia coli Proteins, Hydrolysis, Kinetics, N-Glycosyl Hydrolases, Substrate Specificity, Thionucleosides |
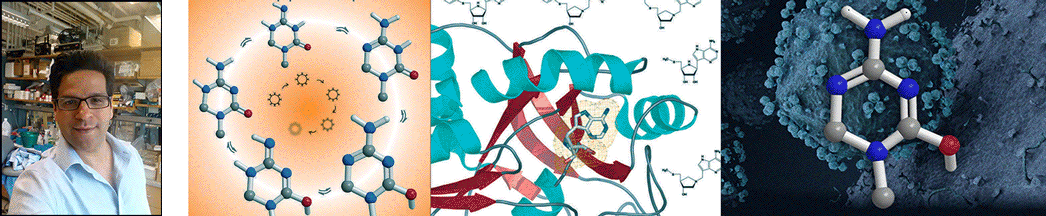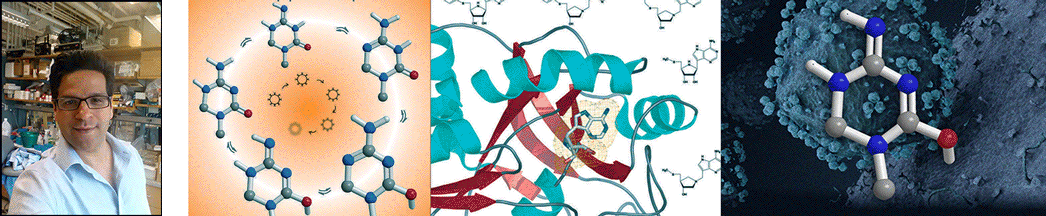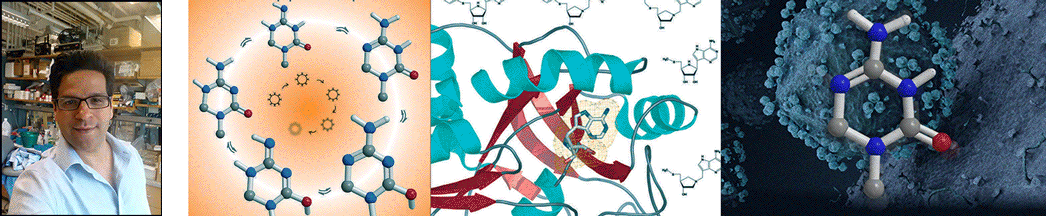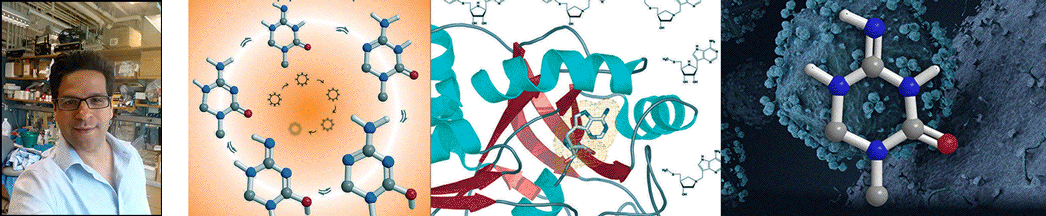
| Abstract | Escherichia coli 5'-methylthioadenosine/S-adenosyl-homocysteine nucleosidase (MTAN) hydrolyzes its substrates to form adenine and 5-methylthioribose (MTR) or S-ribosylhomocysteine (SRH). 5'-Methylthioadenosine (MTA) is a by-product of polyamine synthesis and SRH is a precursor to the biosynthesis of one or more quorum sensing autoinducer molecules. MTAN is therefore involved in quorum sensing, recycling MTA from the polyamine pathway via adenine phosphoribosyltransferase and recycling MTR to methionine. Hydrolysis of MTA by E. coli MTAN involves a highly dissociative transition state with ribooxacarbenium ion character. Iminoribitol mimics of MTA at the transition state of MTAN were synthesized and tested as inhibitors. 5'-Methylthio-Immucillin-A (MT-ImmA) is a slow-onset tight-binding inhibitor giving a dissociation constant (K(i)(*)) of 77 pm. Substitution of the methylthio group with a p-Cl-phenylthio group gives a more powerful inhibitor with a dissociation constant of 2 pm. DADMe-Immucillins are better inhibitors of E. coli MTAN, since they are more closely related to the highly dissociative nature of the transition state. MT-DADMe-Immucillin-A binds with a K(i)(*) value of 2 pm. Replacing the 5'-methyl group with other hydrophobic groups gave 17 transition state analogue inhibitors with dissociation constants from 10(-12) to 10(-14) m. The most powerful inhibitor was 5'-p-Cl-phenylthio-DADMe-Immucillin-A (pClPhT-DADMe-ImmA) with a K(i)(*) value of 47 fm (47 x 10(-15) m). These are among the most powerful non-covalent inhibitors reported for any enzyme, binding 9-91 million times tighter than the MTA and SAH substrates, respectively. The inhibitory potential of these transition state analogue inhibitors supports a transition state structure closely resembling a fully dissociated ribooxacarbenium ion. Powerful inhibitors of MTAN are candidates to disrupt key bacterial pathways including methylation, polyamine synthesis, methionine salvage, and quorum sensing. The accompanying article reports crystal structures of MTAN with these analogues. |
| DOI | 10.1074/jbc.M414472200 |
| Alternate Journal | J Biol Chem |
| PubMed ID | 15749708 |
Femtomolar transition state analogue inhibitors of 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase from Escherichia coli.
Submitted by Vipender Singh on