| Title | Picomolar inhibitors as transition-state probes of 5'-methylthioadenosine nucleosidases. |
| Publication Type | Journal Article |
| Year of Publication | 2007 |
| Authors | Gutierrez JA, Luo M, Singh V, Li L, Brown RL, Norris GE, Evans GB, Furneaux RH, Tyler PC, Painter GF, Lenz DH, Schramm VL |
| Journal | ACS Chem Biol |
| Volume | 2 |
| Issue | 11 |
| Pagination | 725-34 |
| Date Published | 2007 Nov 20 |
| ISSN | 1554-8937 |
| Keywords | Anti-Bacterial Agents, Bacterial Proteins, Enzyme Inhibitors, Isotopes, Molecular Mimicry, Molecular Probes, Protein Binding, Protein Conformation, Purine-Nucleoside Phosphorylase |
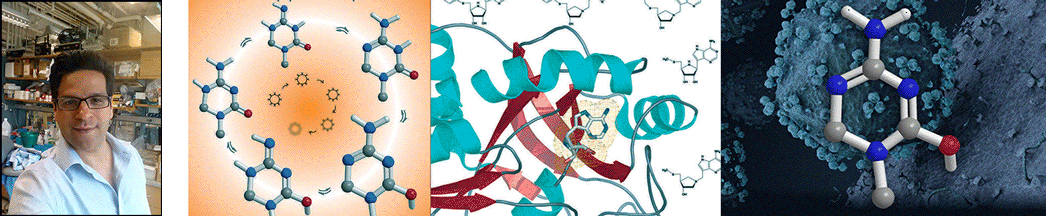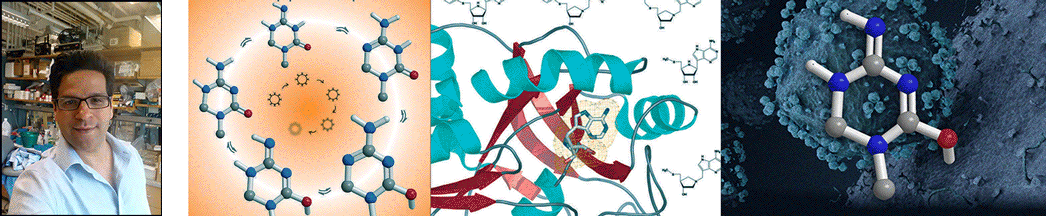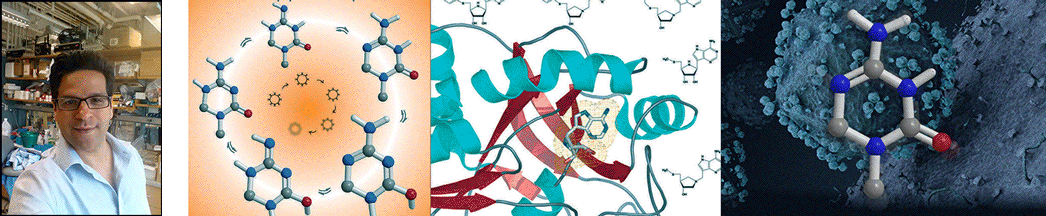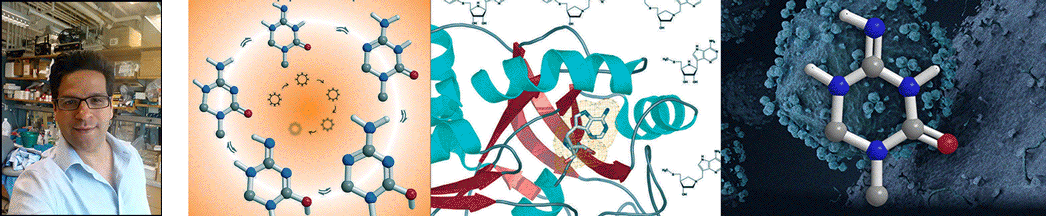
| Abstract | Transition states can be predicted from an enzyme's affinity to related transition-state analogues. 5'-Methylthioadenosine nucleosidases (MTANs) are involved in bacterial quorum sensing pathways and thus are targets for antibacterial drug design. The transition-state characteristics of six MTANs are compared by analyzing dissociation constants (K(d)) with a small array of representative transition-state analogues. These inhibitors mimic early or late dissociative transition states with K(d) values in the picomolar range. Our results indicate that the K(d) ratio for mimics of early and late transition states are useful in distinguishing between these states. By this criterion, the transition states of Neisseria meningitides and Helicobacter pylori MTANs are early dissociative, whereas Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniae, and Klebsiella pneumoniae MTANs have late dissociative characters. This conclusion is confirmed independently by the characteristic [1'- (3)H] and [1'- (14)C] kinetic isotope effects (KIEs) of these enzymes. Large [1'- (3)H] and unity [1'- (14)C] KIEs are observed for late dissociative transition states, whereas early dissociative states showed close-to-unity [1'- (3)H] and significant [1'- (14)C] KIEs. K d values of various MTANs for individual transition-state analogues provide tentative information about transition-state structures due to varying catalytic efficiencies of enzymes. Comparing K d ratios for mimics of early and late transition states removes limitations inherent to the enzyme and provides a better predictive tool in discriminating between possible transition-state structures. |
| DOI | 10.1021/cb700166z |
| Alternate Journal | ACS Chem Biol |
| PubMed ID | 18030989 |
| Grant List | GM 41916 / GM / NIGMS NIH HHS / United States |
Picomolar inhibitors as transition-state probes of 5'-methylthioadenosine nucleosidases.
Submitted by Vipender Singh on